1925 Wolfgang Pauli
One of the most fundamental principles in quantum chemistry and atomic structure is the Pauli Exclusion Principle, formulated by Austrian physicist Wolfgang Pauli in 1925. This principle is crucial in explaining the electronic structure of atoms, the arrangement of electrons in orbitals, and ultimately the behavior of matter at the microscopic level.
What is the Pauli Exclusion Principle?
The Pauli Exclusion Principle states that no two electrons in the same atom can have the same set of all four quantum numbers.
Let’s look at an exceptional example.
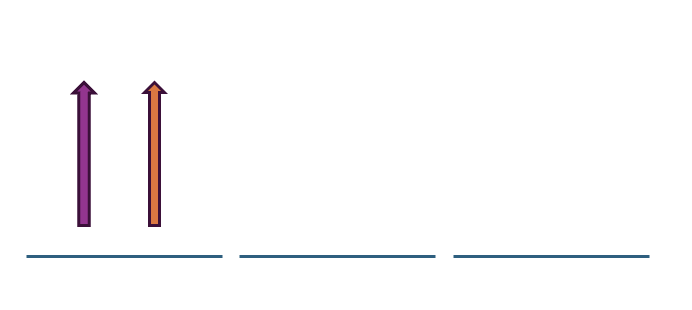
When considered 2px orbital, the values n = 2, l = 1 , ml = -1 , ms = + 1/2 are considered to be purple and orange, and the electrons have the same four quantum numbers.
What is different from the Hund’s rule
One key difference between the Pauli Exclusion Principle and Hund’s Rule lies in the fundamental nature of their restrictions on electron configurations. The Pauli Exclusion Principle states that no two electrons in the same atom can have the same set of four quantum numbers. This means that in any given orbital, a maximum of two electrons can exist, and they must have opposite spins. Violating this principle is not just energetically unfavorable—it is physically impossible in nature. Such a state cannot exist.
On the other hand, Hund’s Rule describes the most stable arrangement of electrons within degenerate orbitals (orbitals of the same energy). According to this rule, electrons occupy separate orbitals with parallel spins as far as possible to minimize electron-electron repulsion. While breaking Hund’s Rule leads to an excited or less stable state, such arrangements can still physically exist in nature, particularly in excited states of atoms or molecules.
In short, violating Hund’s Rule results in a higher-energy state, but violating the Pauli Exclusion Principle results in an impossible state that cannot exist in the universe.