What is Hund’s Rule?
Imagine you get on a bus, and there are plenty of empty seats. Most people prefer to sit alone, leaving an empty seat between themselves and others. We naturally avoid sitting right next to someone when there’s space elsewhere. This is a simple way to understand Hund’s Rule in chemistry.
Hund’s Rule explains how electrons fill orbitals inside atoms. Just like people on a bus, electrons prefer to occupy separate “seats” (orbitals) when possible. In a set of orbitals that have the same energy—called degenerate orbitals—electrons will spread out one by one before pairing up.
In more scientific words:
- Electrons enter degenerate orbitals singly first.
- These single electrons will all have the same spin direction (same spin quantum number, ms)
What if it violates the Hund’s rule
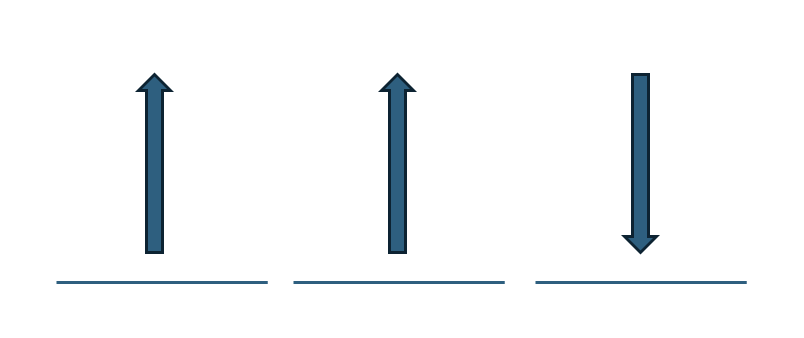
According to Hund’s Rule, electrons must occupy degenerate orbitals singly with the same spin (identical ms) before pairing occurs. This arrangement leads to the most stable and lowest-energy configuration, which is called the ground state.
In the diagram above, we can see a situation that violates Hund’s Rule. Two electrons occupy separate orbitals with parallel spins, but the third electron is placed in an orbital with an opposite spin (different ms value). This creates an excited state, not the ground state.
While this configuration is possible—because electrons can absorb energy and jump to different states—it is less stable and does not follow Hund’s Rule. The violation happens because the spins are not aligned (one ms is different), which increases electron repulsion and destabilizes the atom.
✅ Hund’s Rule satisfied → Ground state
❌ Hund’s Rule violated → Excited state