When a molecule absorbs light, it may emit light through fluorescence or phosphorescence, two types of luminescence with distinct mechanisms and timescales.
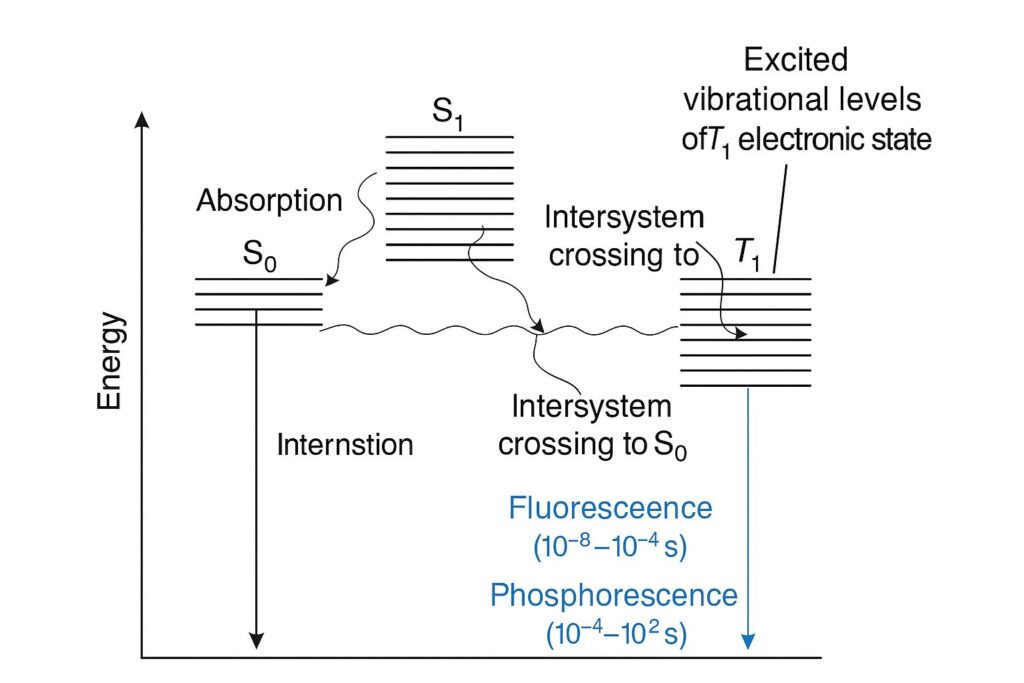
Fluorescence occurs when a molecule absorbs a photon and is promoted to an excited singlet state (S₁). After rapid nonradiative relaxation to the lowest vibrational level of S₁, it emits a photon as it returns to the ground singlet state (S₀). This process is fast—typically between 10⁻⁸ and 10⁻⁴ seconds.
In contrast, phosphorescence involves a forbidden transition. After intersystem crossing from S₁ to the triplet state T₁, the molecule eventually emits a photon as it transitions from T₁ to S₀. Since this involves a change in electron spin, it is slower—ranging from 10⁻⁴ to 10² seconds, often requiring cooling to be observable.
The emitted light in phosphorescence is of lower energy (i.e., longer wavelength) than in fluorescence, due to greater energy loss during internal conversion and intersystem crossing. Both processes are commonly used in analytical chemistry, fluorescent labeling, and even laundry detergents that make clothes appear whiter under UV light
Quiz
· Fluorescence is a transition from the _______ excited state to the ground state.
· Phosphorescence involves a transition from the _______ state to the ground singlet state.
· The timescale of phosphorescence is _______ than that of fluorescence.
· A nonradiative transition between states of the same spin is called _______.
· Emission of light during phosphorescence occurs on the order of _______ seconds.
Answer
signlet , triplet , longer , internal conversion , 10⁻⁴ to 10²